A phase 3 cluster Randomized Control Trial was done in an area of intense resistance and malaria transmission in Ivory Coast between 2016 and 2019. All 40 villages received LLINs, and 3022 houses in 20 intervention villages received in total over 30.000 In2Care EaveTubes and window screening. The trial was executed by researchers from Penn State University, the London School of Hygiene and Tropical Medicine (LSHTM), and the local Institute Pierre Richet in Ivory Coast, in close collaboration with In2Care. The trial was funded by the Bill and Melinda Gates Foundation. Results are published in medical journal The Lancet.

Results showed a significant impact on malaria transmission:
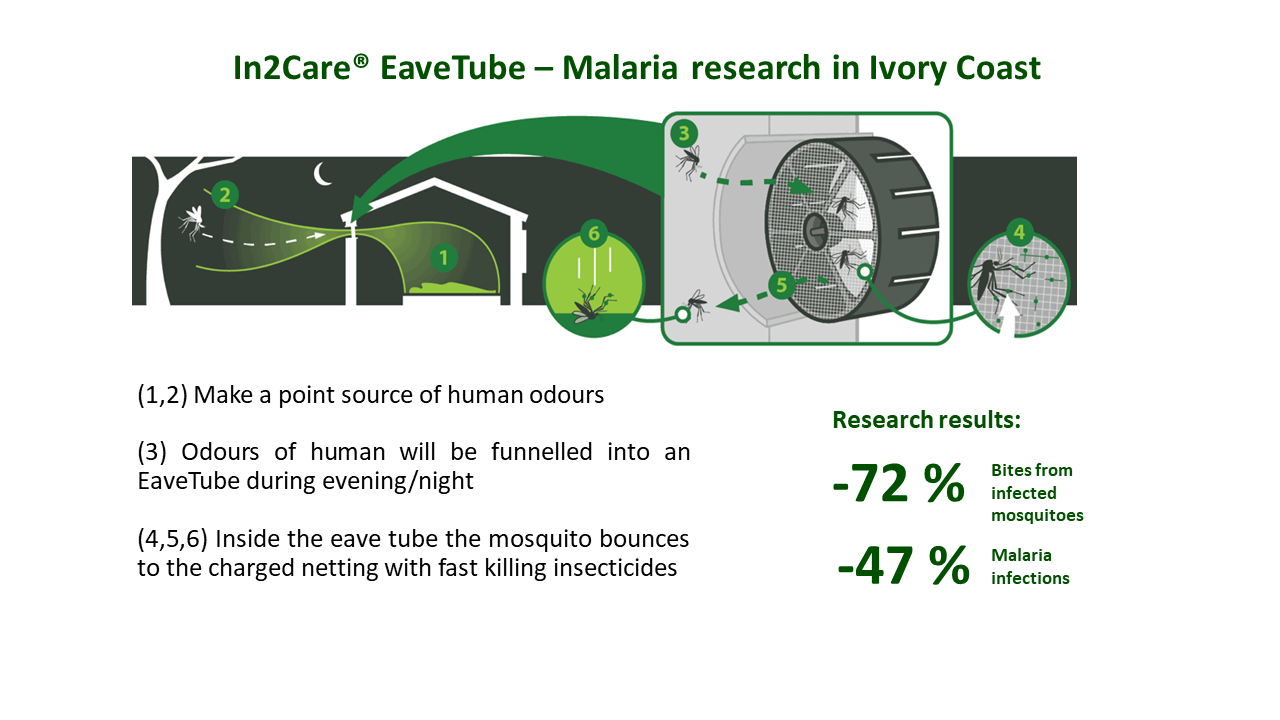
- On top of bednets, EaveTubes reduce malaria on average with 38% and up to 47% in villages where 70% of the houses were treated with EaveTubes and window screening.
- 10% increase in EaveTube coverage result in 10% decrease in malaria incidence.
- The In2Care intervention is creating a community effect: even for children living in houses without EaveTubes, risk of malaria is lower.
- Odds of anaemia in children living in villages with EaveTubes was 30% lower.
- In2Care EaveTubes reduced indoor vector densities with 39% and lowered the infectious mosquito bites (EIR) with 72%.
- Social studies showed >90% of inhabitants liking the effects and wanting to keep using In2Care EaveTubes.

The work with EaveTubes in Ivory Coast is still ongoing. Houses will be revisited, and the netting of the tubes will be replaced. Execution will take place by EaveTube Paludism, a local company in Ivory Coast, supported by In2Care. As product developer and IP owner, In2Care is preparing mass-scale Eave Tube manufacturing processes and developing sustainable business models for Eave Tube commercialization. In2Care’s main objective is to continue protecting people against malaria and other diseases transmitted by mosquitoes.


